All wines are bubbly at some point because carbon dioxide is a byproduct of fermenting sugar into alcohol. However, this natural carbonation is usually allowed to dissipate, leaving a still wine behind.
Why? It is much easier to allow carbon dioxide to escape than to preserve it. Finding a means to preserve carbonation is a tremendous challenge, since wine must be kept under pressure continuously at every step of production, transport and storage.
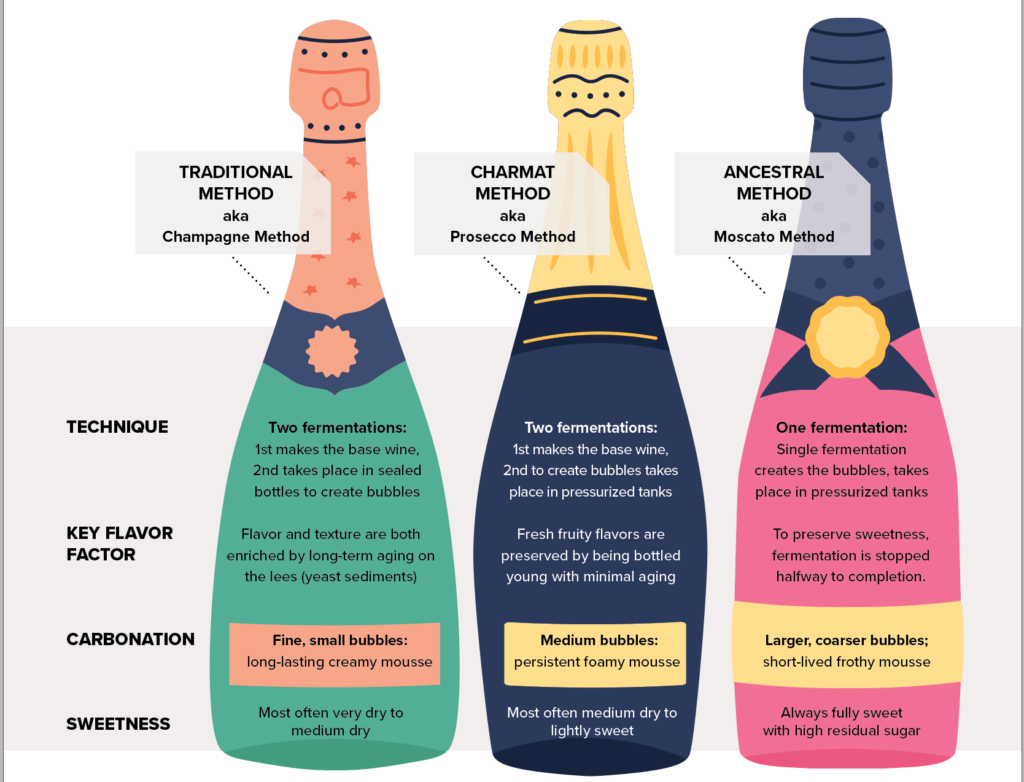
While wines that “sparkled” were first commercialized in the 16th century, glass bottles strong enough to withstand the internal pressure of a second fermentation were not manufactured at scale until the Industrial Revolution. “Sparkling wine” as we know it did not catch on until the early 19th century, pioneered in the Champagne region of France.
Today, virtually all fine sparkling wine is made following the procedures developed in Champagne, now called the “Traditional Method,” where a second fermentation in bottle traps the wine’s carbonation. More modern methods that rely on pressurized tanks are used for making less-prestigious sparklers, like Prosecco and Moscato, making them far less expensive to produce.
Feature photo by Marianne Gamet on Unsplash.
Marnie Old is one of the country’s leading wine educators. Formerly the director of wine studies for Manhattan’s French Culinary Institute, she currently serves as director of vinlightenment for Boisset Collection and is best known for her visually engaging books published by DK – the award-winning infographic Wine: A Tasting Course for beginners and the tongue-in-cheek He Said Beer, She Said Wine. Read her recent piece, Sorting Wine Grapes Into Flavor Families.





love the education made simple
i am a wine importer and love the way you described the prosses.